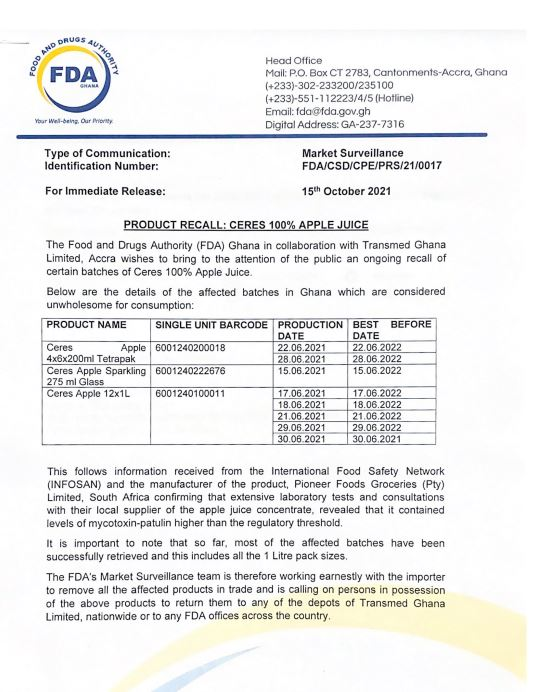
The Food and Drugs Authority (FDA) is recalling certain batches of Ceres apple juice from the market.
The products include the Ceres Apple Tetrapack, Apple sparkling, and Ceres Apple 12x1L.
The outfit explains the recall follows information that the products contain high levels of mycotoxin-patulin, a food mould, above the regulatory threshold.
The FDA, in a statement that announced the recall on Friday, said it has so far retrieved most of the affected batches from the market.
However, the outfit has appealed to the general public to help to retrieve the rest.
The FDA has since urged the public to return all of the affected products in their possession to the depots of Transmed Ghana Limited or any of the FDA’s offices nationwide.
Read the full statement below: